Patients’ Experience with Dr. Williams
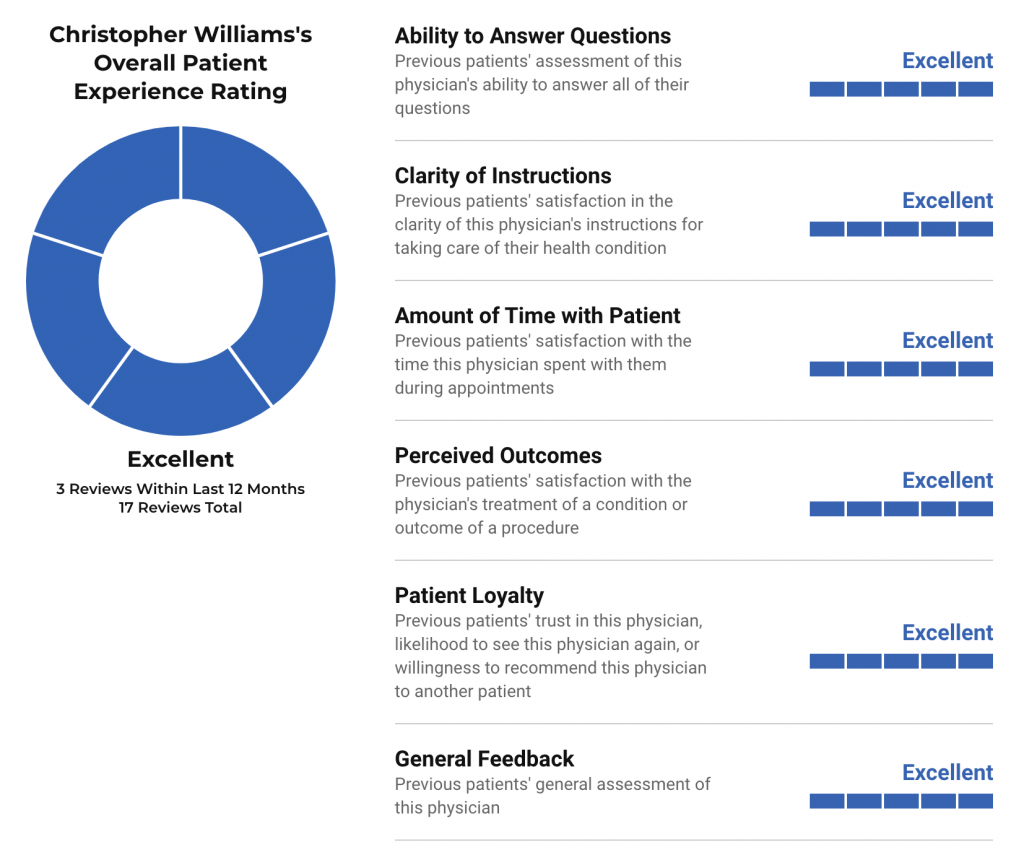
When choosing a doctor, it can be helpful to review other patients’ experiences. U.S. News publishes patient experience ratings from Binary Fountain, which aggregates patient reviews from over a hundred sites to compile information about 10 different patient experience metrics. These ratings are not intended as indicators of medical quality — how good a doctor is — but rather reflect patients’ feedback on factors such as good communication, clarity of instructions, etc. Patient experience ratings are issued in the context of a specific physician’s specialty, as patients tend to rate types of doctors differently.
Christopher Williams’s Overall Patient Experience Rating
Education & Experience
College & Residency
University of Alabama Medical Center- Residency , General Surgery
University of Alabama Medical Center-Residency , Urology
National Cancer Institute- Fellowship , Urological Oncology
University of North Carolina at Chapel Hill School of Medicine-Medical School
Certifications & Licensure
American Board of Urology
Certified in Urology
FL State Medical License
Active through 2020
Publications
- Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas.Miller, C.R., Williams, C.R., Buchsbaum, D.J., Gillespie, G.Y.
- Challenges and potential solutions to meeting accrual goals in a Phase II chemoprevention trial for prostate cancer.Kumar, N., Crocker, T., Smith, T., Pow-Sang, J., Spiess, P.E., Egan, K., Quinn, G., Schell, M., Sebti, S., Kazi, A., Chuang, T., Salup, R., Helal, M., Zagaja, G., Trabulsi, E., McLarty, J., Fazili, T., Williams, C.R., Schreiber, F., Slaton, J., Anderson,
- Quality of life of patients undergoing surgical treatment for newly-diagnosed, clinically localized renal cell carcinoma.Ames, S.C., Parker, A.S., Crook, J.E., Diehl, N.N., Tan, W.W., Williams, C.R., Ames, G.E.
- Proton Radiotherapy for Prostate Cancer Is Not Associated With Post-Treatment Testosterone Suppression.Nichols, R.C., Morris, C.G., Hoppe, B.S., Henderson, R.H., Marcus, R.B., Mendenhall, W.M., Li, Z., Williams, C.R., Costa, J.A., Mendenhall, N.P.
- Early Outcomes from Three Prospective Trials of Image-guided Proton Therapy for Prostate Cancer.Mendenhall, N.P., Li, Z., Hoppe, B.S., Marcus, R.B., Mendenhall, W.M., Nichols, R.C., Morris, C.G., Williams, C.R., Costa, J., Henderson, R.
- Intratumor injection of the Hsp90 inhibitor 17AAG decreases tumor growth and induces apoptosis in a prostate cancer xenograft model.Williams, C.R., Tabios, R., Linehan, W.M., Neckers, L.
- Highlights from the Society of Urologic Oncology, 5th Annual Meeting, December 3-4, 2004, Bethesda, Maryland, United States of America.Grubb, R.L., Behari, A., Kim, C.M., Williams, C.R., Linehan, W.M., Coleman, J.A.
- Metastatic renal cell carcinoma to contralateral ureter presenting as acute obstructive renal failure after radical nephrectomy.
- Testicular torsion: is there a seasonal predilection for occurrence?Williams, C.R., Heaven, K.J., Joseph, D.B.
- Hemorrhagic Radiation Cystitis.Mendenhall, W. M.,Henderson, R. H.,Costa, J. A.,Hoppe, B. S.,Dagan, R.,Bryant, C. M.,Nichols, R. C.,Williams, C. R.,Harris, S. E.,Mendenhall, N. P.
- Does Race Influence Health-related Quality of Life and Toxicity Following Proton Therapy for Prostate Cancer?Bryant, C.,Mendenhall, N. P.,Henderson, R. H.,Nichols, R. C.,Mendenhall, W. M.,Morris, C. G.,Williams, C.,Su, Z.,Li, Z.,Hoppe, B. S.
- Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer.Mendenhall, N. P.,Hoppe, B. S.,Nichols, R. C.,Mendenhall, W. M.,Morris, C. G.,Li, Z.,Su, Z.,Williams, C. R.,Costa, J.,Henderson, R. H.
- Comparative effectiveness study of patient-reported outcomes after proton therapy or intensity-modulated radiotherapy for prostate cancer.Hoppe, B. S.,Michalski, J. M.,Mendenhall, N. P.,Morris, C. G.,Henderson, R. H.,Nichols, R. C.,Mendenhall, W. M.,Williams, C. R.,Regan, M. M.,Chipman, J. J.,Crociani, C. M.,Sandler, H. M.,Sanda, M. G.,Hamstra, D. A.
- The combination of an mTORc1/TORc2 inhibitor with lapatinib is synergistic in bladder cancer in vitro.Becker, M. N.,Wu, K. J.,Marlow, L. A.,Kreinest, P. A.,Vonroemeling, C. A.,Copland, J. A.,Williams, C. R.
- Hypofractionated passively scattered proton radiotherapy for low- and intermediate-risk prostate cancer is not associated with post-treatment testosterone suppression.Kil, W. J.,Nichols, R. C.,Hoppe, B. S.,Morris, C. G.,Marcus, R. B.,Mendenhall, W.,Mendenhall, N. P.,Li, Z.,Costa, J. A.,Williams, C. R.,Henderson, R. H.
- Outcomes in men with large prostates (? 60 cm(3)) treated with definitive proton therapy for prostate cancer.McGee, L.,Mendenhall, N. P.,Henderson, R. H.,Morris, C. G.,Nichols, R. C.,Marcus, R. J.,Li, Z.,Mendenhall, W. M.,Williams, C. R.,Hoppe, B. S.
- Urinary functional outcomes and toxicity five years after proton therapy for low- and intermediate-risk prostate cancer: Results of two prospective trials.Henderson, R. H.,Hoppe, B. S.,Marcus, R. B.,Mendenhall, W. M.,Nichols, R. C.,Li, Z.,Su, Z.,Morris, C. G.,Williams, C. R.,Costa, J.,Mendenhall, N. P.
- Hip fractures and pain following proton therapy for management of prostate cancer.Valery, R.,Mendenhall, N. P.,Nichols, R. C.,Henderson, R.,Morris, C. G.,Su, Z.,Mendenhall, W. M.,Williams, C. R.,Li, Z.,Hoppe, B. S.
- Urethral catheterization facilitates preradiation fiducial marker placement in postprostatectomy patients.Williams, C., Costa, J., Mandia, S., Henderson, R., Marino, R., Mendenhall, N.
- Erectile function, incontinence, and other quality of life outcomes following proton therapy for prostate cancer in men 60 years old and younger.Hoppe, B.S., Nichols, R.C., Henderson, R.H., Morris, C.G., Williams, C.R., Costa, J., Marcus, R.B., Mendenhall, W.M., Li, Z., Mendenhall, N.P.
- Serum Testosterone 60 Months after Passive-Scatter Proton Therapy for Localized Prostate Cancer.Nichols, R. C.,Morris, C. G.,Bryant, C.,Hoppe, B. S.,Henderson, R. H.,Mendenhall, W. M.,Li, Z.,Costa, J. A.,Williams, C. R.,Mendenhall, N. P.
- Rectal Culture and Sensitivity Analysis for Reducing Sepsis Risk After Fiducial Marker Placement.Mendenhall, W. M., Mendenhall, W. M., Sarto, G., Sarto, G., Bryant, C. M., Bryant, C. M., Morris, C. G., Morris, C. G., Williams, C. R., Williams, C. R., Costa, J. A., Costa, J. A., Bandyk, M., Bandyk, M., Hoppe, B. S., Hoppe, B. S., Henderson, R. H., Henderson, R. H., Nichols, R. C., Nichols, R. C., Mendenhall, N. P., Mendenhall, N. P.
- Long-term supplementation of decaffeinated green tea extract does not modify body weight or abdominal obesity in a randomized trial of men at high risk for prostate cancer.Kumar, N. B.,Patel, R.,Pow-Sang, J.,Spiess, P. E.,Salup, R.,Williams, C. R.,Schell, M. J.
- Five-Year Biochemical Results, Toxicity, and Patient-Reported Quality of Life After Delivery of Dose-Escalated Image Guided Proton Therapy for Prostate Cancer.Bryant, C., Bryant, C., Smith, T. L., Smith, T. L., Henderson, R. H., Henderson, R. H., Hoppe, B. S., Hoppe, B. S., Mendenhall, W. M., Mendenhall, W. M., Nichols, R. C., Nichols, R. C., Morris, C. G., Morris, C. G., Williams, C. R., Williams, C. R., Su, Z., Su, Z., Li, Z., Li, Z., Lee, D., Lee, D., Mendenhall, N. P., Mendenhall, N. P.
- Patient-Reported Quality of Life in Men with Transurethral Resection of the Prostate Undergoing Proton Therapy for Management of Prostate Cancer.Lee, D. T.,Mendenhall, N. P.,Smith, T. L.,Morris, C. G.,Nichols, R. C.,Bryant, C.,Henderson, R. H.,Mendenhall, W. M.,Costa, J.,Williams, C. R.,Li, Z.,Hoppe, B. S.
- Proton Therapy as Salvage Treatment for Local Relapse of Prostate Cancer Following Cryosurgery or High-Intensity Focused Ultrasound.Holtzman, A. L., Hoppe, B. S., Letter, H. P., Bryant, C., Nichols, R. C., Henderson, R. H., Mendenhall, W. M., Morris, C. G., Williams, C. R., Li, Z., Mendenhall, N. P.
- En Bloc Robot-assisted Laparoscopic Partial Cystectomy, Urachal Resection, and Pelvic Lymphadenectomy for Urachal Adenocarcinoma.Williams, C. R., Chavda, K.
- Randomized, Placebo-Controlled Trial of Green Tea Catechins for Prostate Cancer Prevention.Kumar, N. B., Kumar, N. B., Kumar, N. B., Pow-Sang, J., Pow-Sang, J., Pow-Sang, J., Egan, K. M., Egan, K. M., Egan, K. M., Spiess, P. E., Spiess, P. E., Spiess, P. E., Dickinson, S., Dickinson, S., Dickinson, S., Salup, R., Salup, R., Salup, R., Helal, M., Helal, M., Helal, M., McLarty, J., McLarty, J., McLarty, J., Williams, C. R., Williams, C. R., Williams, C. R., Schreiber, F., Schreiber, F., Schreiber, F., Parnes, H. L., Parnes, H. L., Parnes, H. L., Sebti, S., Sebti, S., Sebti, S., Kazi, A., Kazi, A., Kazi, A., Kang, L., Kang, L., Kang, L., Quinn, G., Quinn, G., Quinn, G., Smith, T., Smith, T., Smith, T., Yue, B., Yue, B., Yue, B., Diaz, K., Diaz, K., Diaz, K., Chornokur, G., Chornokur, G., Chornokur, G., Crocker, T., Crocker, T., Crocker, T., Schell, M. J., Schell, M. J., Schell, M. J.
- Prostate Cancer Chemoprevention Targeting Men with High-Grade Prostatic Intraepithelial Neoplasia (HGPIN) and Atypical Small Acinar Proliferation (ASAP): Model for Trial Design and Outcome Measures.Kumar, N., Crocker, T., Smith, T., Connors, S., Pow-Sang, J., Spiess, P. E., Egan, K., Quinn, G., Schell, M., Sebti, S., Kazi, A., Chuang, T., Salup, R., Helal, M., Zagaja, G., Trabulsi, E., McLarty, J., Fazili, T., Williams, C. R., Schreiber, F., Anderson, K.
